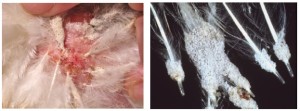
Common Lice and Mites of Poultry: Identification and Treatment
Lice are ectoparasites and spend their entire life on an animal host. Most louse species are host specific, meaning that they can feed on only one or a few closely related species of animal hosts. Poultry lice cannot survive on humans or on our non-bird domestic pets. In fact, a poultry louse generally completes its entire life cycle from egg to adult on a single bird, and will die within a few days to a week if separated from a host. The number of lice on poultry tend to be greatest during the autumn and winter.

 Size of air cell on 7th, 14th and 18th day of incubation
Size of air cell on 7th, 14th and 18th day of incubation